The interactions between mineral cations and anions are well understood by chemists, but often overlooked by horticulturists when applying fertilizers to tailor the growth of a specific plant variety.
You may have heard the phrase “It’s not what you eat, it’s what you absorb,” a phrase that applies equally to the uptake of essential nutrients by plants. Application of an essential plant nutrient does not always mean that the plant will be able to uptake that mineral and then move it through the vascular system into the plant tissues.
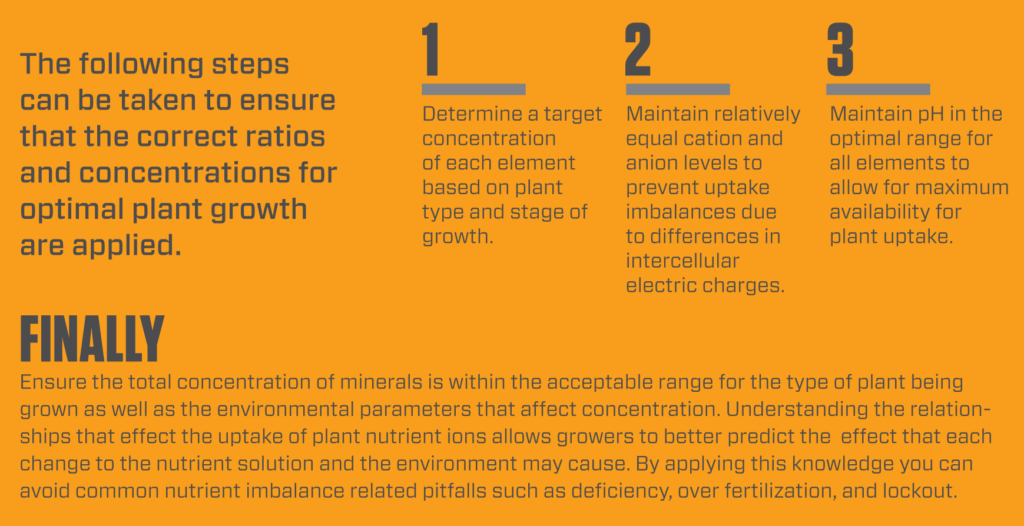
The availability of plant nutrients is in fact dictated by the form of mineral, environmental temperature, humidity, photosynthesis, pH of the root zone, and most importantly the relative concentration of each mineral in the nutrient solution. It is the balance of these minerals that is often forgotten when growers are formulating plant nutrient recipes and adding supplements to reach specific targeted mineral compositions.
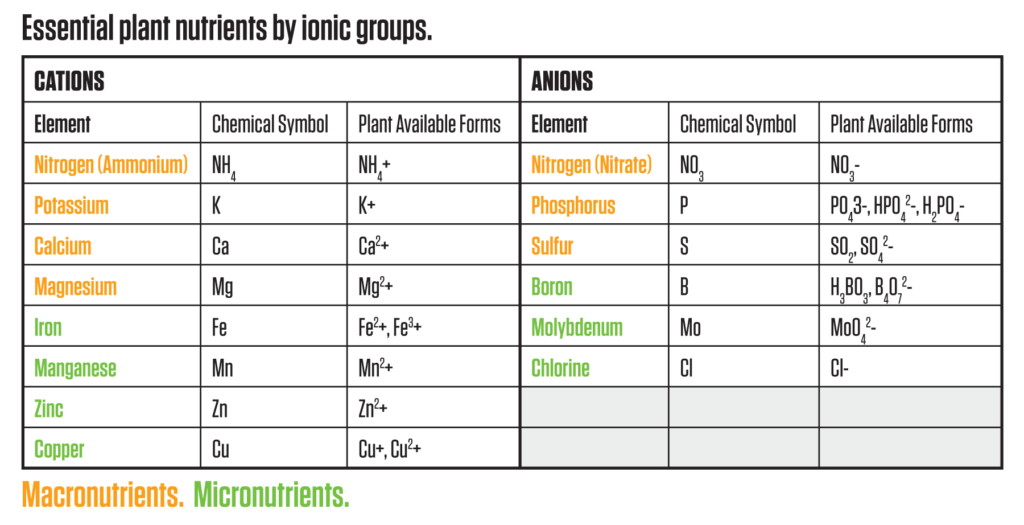
There is a well-known system that classifies essential plant nutrients into “macro” and “micro” categories based on their concentrations in the plant tissue. Less understood is the relationship of the electrical charge of the individual ions and how it affects their bioavailability to the plant. Ions exist as either positively charged (cations) or negatively charged (anions) depending on the balance of electrons (negative) versus protons (positive).
It is the strength of the ionic charge that will affect the movement of the ions into and out of the plant. By understanding the strength of the positive or negative charge of essential plant nutrients, we can begin to comprehend the selective ion uptake mechanisms of a plant’s physiology. The table below shows the elemental forms of plant nutrients and their ionic charges in the forms that are available for plant uptake.
The movement of ions into plant roots occurs by both active and passive transport. Passive transport means that the ions are carried with the uptake of water into the plant without energy from the plant. The water movement factors that affect passive transport are temperature, humidity, photosynthesis rates, concentration of ions in solution versus within the plant cell, and plant transpiration rates based on stage of growth. Active transport requires energy from the plant and ion movement is determined by competition between ions based on their individual charge. The monovalent ions (single charged) are moved into the plant more easily than divalent ions (double charged), while divalent ions are taken up more easily than trivalent ions (triple charged). This means that the plant will accumulate more potassium (a monovalent ion) than calcium and magnesium (divalent ions) due to the difference in their charge.
Plants typically maintain a negative interior (inside the plasma membrane) relative to the exterior. The slightly negative state of the cell interior and the environment must be maintained and, thus, is related to ion uptake. When there are more cations than anions present, the overall charge becomes excessively positive, and an increase in anions or a decrease in cation uptake occurs to restore physiological conditions. For example, an excess of ammonium (NH4+) cations decreases the uptake of potassium (K+), calcium (Ca2+), and Magnesium (Mg2+). The same relationship exists for anions – excess anions lead to a lower uptake of anions or an increase in cations to balance the cell’s charge. If nitrate (NO3-) is the major anion in excess, then the uptake of cations such as potassium (K+), calcium (Ca2+), and Magnesium (Mg2+) will increase to compensate for the overall negative charge caused by excess nitrate levels.
– Brandon Jewell
Continued in Mixing Nutrients – A Beginner’s Guide ⟶